A Sample of Zinc Metal Reacts Completely With
A sample of zinc metal reacts completely with an excess. Magnesium metal reacts with hydrochloric acid to form magnesium chloride and hydrogen gas.

Solved Consider This Reaction Zinc Metal Reacts With Chegg Com
Zns 2 HClaq ZnCl2aq H28 look GO The hydrogen gas.
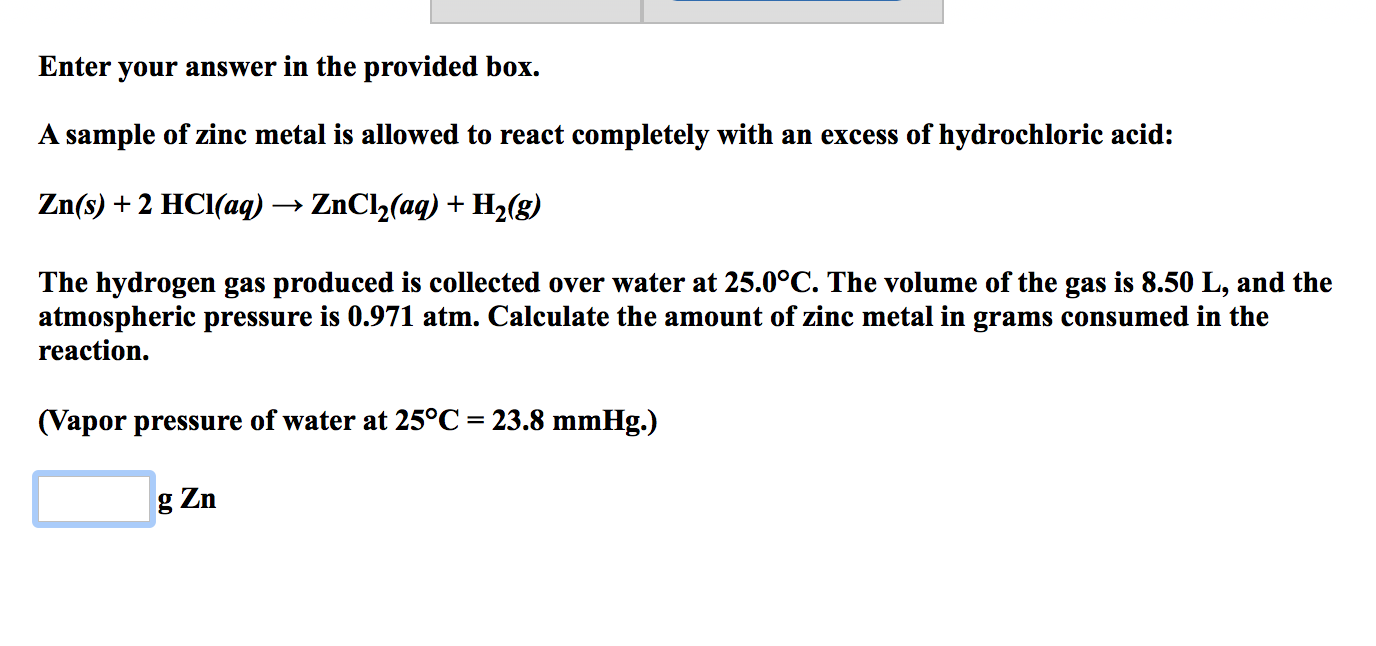
. The volume of the gas is 780 L and the pressure is 0980 atm. A sample of zinc metal reacts completely with an excess of hydrochloric acid. A piece of zinc reacts completely with hydrochloric acid HClaq to produce an aqueous solution of zinc chloride ZnCl2aq and hydrogen gas.
780 L of the gas was collected at a total pressure of 0980 atm. Zns 2 HClaq rightarrow ZnCl_2aq H_2g The hydrogen gas produced is collected over water at 250 degree C. Calculate the mass of zinc metal that reacted.
Up to 256 cash back A sample of zinc metal is allowed to react completely with an excess of hydrochloric acid. MathrmZns2 mathrmHCla q longrightarrow mathrmZnCl_2a qmathrmH_2g The hydrogen gas produced is collected over water at 250circ mathrmC using an arrangement similar to that shown in Figure 1114mathrma The. A sample of zinc metal reacts completely with an excess of hydrochloric acid.
Zn s 2HCl aq --- ZnCl2 aq H2 g The hydrogen gas produced is collected over water at 250 degrees C. A Sample of zinc metal reacts completely with hydrochloric acid. 2g The hydrogen gas is collected over water at 25oC.
SOLVEDA sample of zinc metal reacts completely with an excess of hydrochloric acid. 1 Answer anor277 Apr 29 2017 Approx. Calculate the amount of zinc metal in grams consumed in the reaction.
This equation tells us that one mole of zinc will produce one mole of hydrogen gas. Therefore 00446 moles of zinc will produce 00446 moles of hydrogen. The reaction of zinc metal with hydrochloric acid to produce hydrogen can be represented by the equation-Zn 2HCl ZnCl2 H2.
The gas volume is found to be 780L and the atmospheric pressure is 0980atm. A sample of zinc metal reacts completely with an excess of hydrochloric acid. The volume of the gas is 780 L and the pressure is 0980 atm.
Zns 2HClaq ZnCl. Use the data shown below to determine the enthalpy of reaction per mole of zinc. A sample of zinc metal reacts completely with an excess of hydrochloric acid.
Calculate the amount of zinc metal in grams consumed in the reaction. Z n s 2 H C l a q Z n C l 2 a q H 2 g beginalign mathrmZns2mathrmHClaqrightarrowmathrmZnCl_2aqmathrmH_2g endalign Zn s 2 HCl a q ZnC l 2 a q H 2 g. Mathrm Zn s2 mathrm HCl a q longrightarrow mathrm ZnCl_ 2 a qmathrm H_ 2 g The hydrogen gas produced is collected over water at 250 circ mathrm C using an arrangement similar to that shown in Figure 1114 mathrm a The volume of the gas is 780 mathrm L.
A sample of zinc metal reacts completely with an excess of hydrochloric acid. The hydrogen gas produced is collected over water at 250C. How many grams of solid zinc metal will completely react with 1000 mL of 00525 M HCl if zinc chloride and hydrogen gas are the only products.
Zns 2HClaq ZnCl2aq H2g The hydrogen gas produced is collected over water at 250C using an arrangement similar to that shown in Figure 515. A sample of zinc metal is allowed to react completely with an excess of hydrochloric acid.

Solved Enter Your Answer In The Provided Box A Sample Of Chegg Com

Solved Zinc Metal Reacts With Hydrochloric Acid To Produce Chegg Com
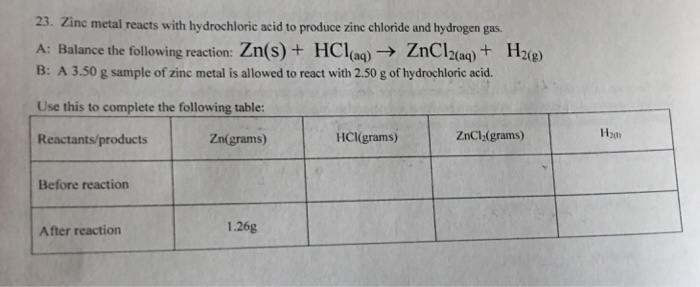
Solved Zinc Metal Reacts With Hydrochloric Acid To Produce Chegg Com
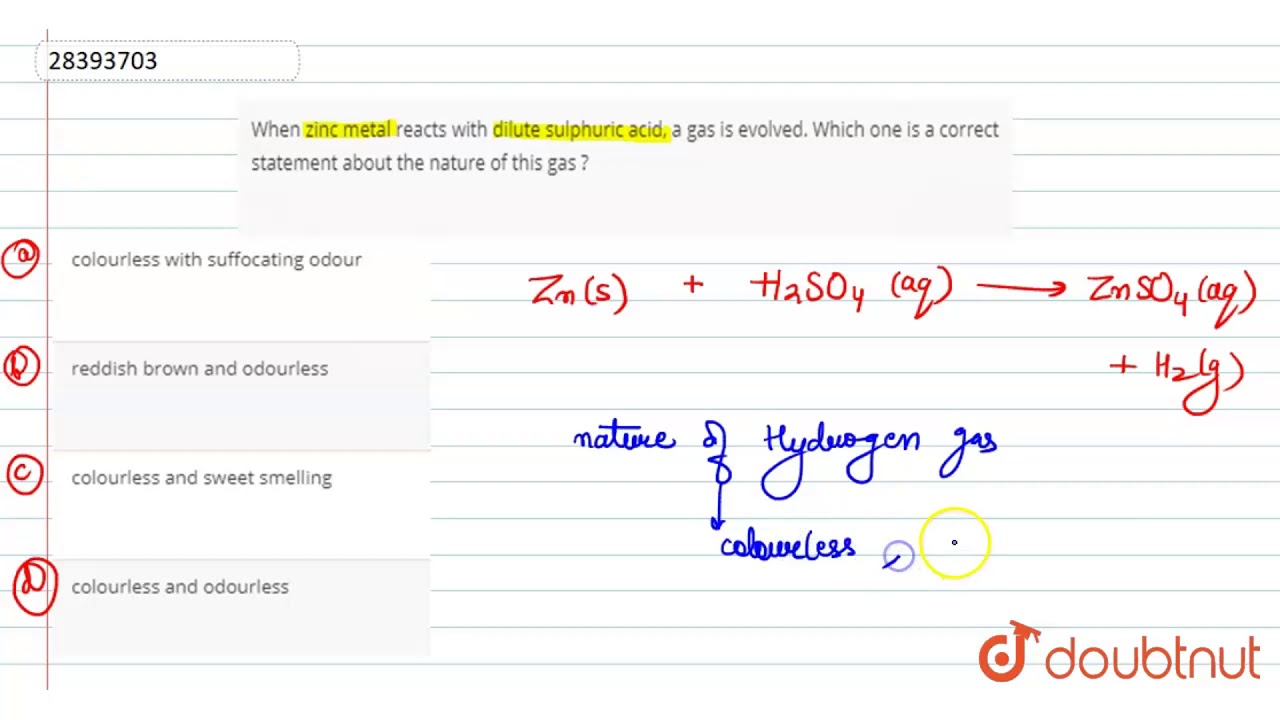
When Zinc Metal Reacts With Dilute Sulphuric Acid A Gas Is Evolved Which One Is A Correct Youtube
Comments
Post a Comment